此前美國再生元制藥公司的研究已證實,生長分化因子-8(GDF-8,又稱肌抑素、MSTN)和激活素 A(Activin A)是介導II型激活素受體(ActRIIA/B)“減少肌肉量” 作用的兩種主要配體[1]。近日,該團隊在Nature Communication(IF15.7)發表文章“GDF8 and activin A blockade protectsagainst GLP-1–induced muscle loss whileenhancing fat loss in obese male mice and non-human primates”(在肥胖雄性小鼠與非人靈長類中GDF-8與activin A雙重阻斷會防止GLP-1誘導的肌肉減少并增強減脂效果),研究表明在人類肥胖治療中,將胰高血糖素樣肽-1 受體激動劑治療(司美格魯肽,GLP-1 RA)與GDF-8、激活素A(Act A)阻斷手段相結合,有望大幅提升減重質量,即減少肌肉流失、增加脂肪消耗[2]。
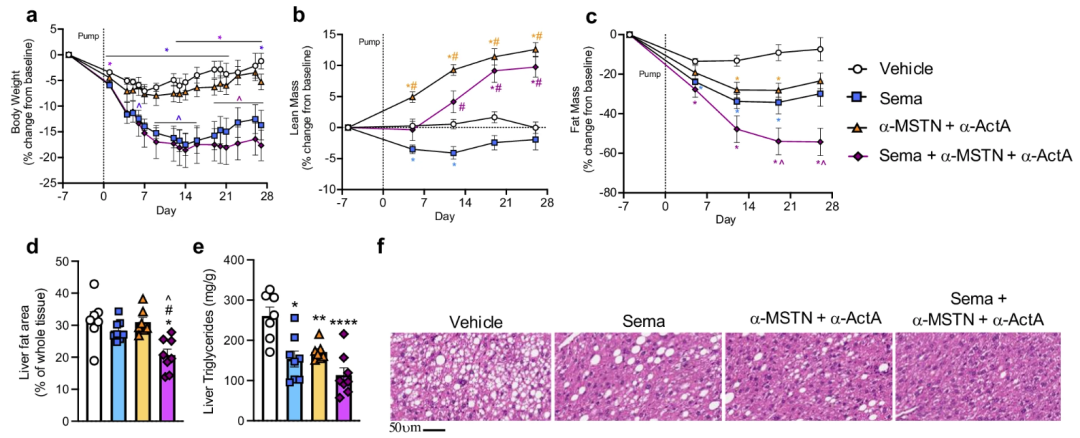
圖1 將α-MSTN/α-ActA與司美格魯肽聯合使用對小鼠身體成分產生了顯著有益的影響[2]
2023年,制藥巨頭禮來(Eli Lilly)為收購Versanis公司支付高達19億美元,其核心目標正是ActRIIA/B單抗藥物Bimagrumab,這一重磅交易充分體現了大型藥企對該靶點治療肥胖和肌少癥前景的極大信心。
關于該靶點的另一明星藥物,Scholar Rock公司的在研GDF-8抑制劑Apitegromab,于2025年9月22日被FDA正式拒絕批準,原因是一家第三方制造商的生產場地存在安全隱患。但FDA未提及包括藥物的療效或安全性缺陷在內的任何其他影響審批的顧慮,這恰恰從側面印證了Apitegromab堅實的臨床數據:其三期研究顯示,8周即可起效、52周持續改善SMA患者運動功能。此次挫折是商業化進程中的可解決問題,反而更凸顯了其作用機制的強大潛力和明確的臨床獲益。
GDF-8靶向療法正從罕見病邁向更廣闊市場,前景依舊可期。近岸蛋白提供經過Cell/ELISA/BLI等活性驗證的高質量GDF-8、Latent GDF-8以及GDF-8報告基因細胞株,適用于動物免疫、抗體篩選、功能評估和質量控制等不同環節的需求。
01 GDF-8靶點簡介
- 基因定位:
人GDF-8基因位于染色體2q32.2上; - 蛋白質分類:
它是一種分泌蛋白,屬于轉化生長因子-β(TGF-β)超家族的成員; - 發現歷史:
其關鍵發現源于1997年的基因工程小鼠研究。科學家通過基因敲除技術使GDF8基因失活,結果發現小鼠的肌肉質量出現了驚人的倍增,因此被形象地稱為“超級肌肉”小鼠。這一現象直接證明了GDF-8的核心功能是作為肌肉生長的負向調控因子[3]。 - 蛋白前體的結構:
與其他TGF-β家族成員一樣,GDF-8最初以一種前體蛋白的形式合成,經過弗林蛋白酶(furin proteases)的加工,生成一個N端前肽和一個C端肽段,這兩個肽段通過二硫鍵連接形成的二聚體才是真正的信號分子[4]。通過進化選擇,GDF-8的序列高度保守,其成熟形式的氨基酸序列在人類和火雞等差異極大的物種中完全相同[5]。
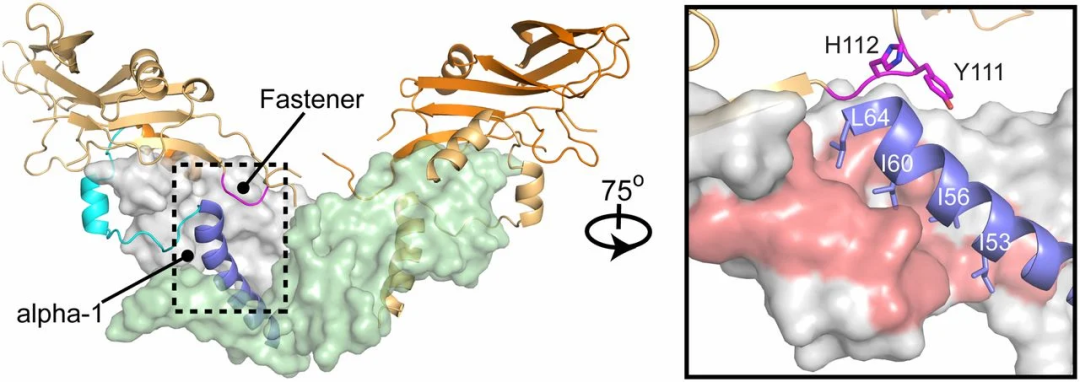
圖2 GDF-8前體結構域復合物的晶體結構[6]。該復合物包含成熟的二聚體(灰色和淺綠色)、α-1螺旋(藍色)、潛伏環(青色)以及扣鎖結構(品紅色)。(中插圖)展示了沿y軸旋轉75°后的α-1螺旋與扣鎖區域的結構(中部)。
02 GDF-8靶點作用機制揭秘
遺傳學研究表明,GDF-8作為肌肉質量負調節劑的功能在進化過程中得到了高度保守[4]。除了在調控肌纖維數量和組成方面的發育作用外,還調控肌纖維的生長[7,8]。GDF-8以前體形式合成,經蛋白酶切后仍與其前肽結合,處于潛活狀態;該復合物可被BMP-1/TLL等蛋白酶激活,其活性亦受GASP、卵泡抑素等胞外抑制蛋白調控。激活后的GDF-8與激活素II型受體(ACVR2/ACVR2B)結合,進而招募I型受體ALK4/ALK5,磷酸化SMAD2/3并與SMAD4形成復合物,轉入細胞核抑制肌肉生長基因轉錄。
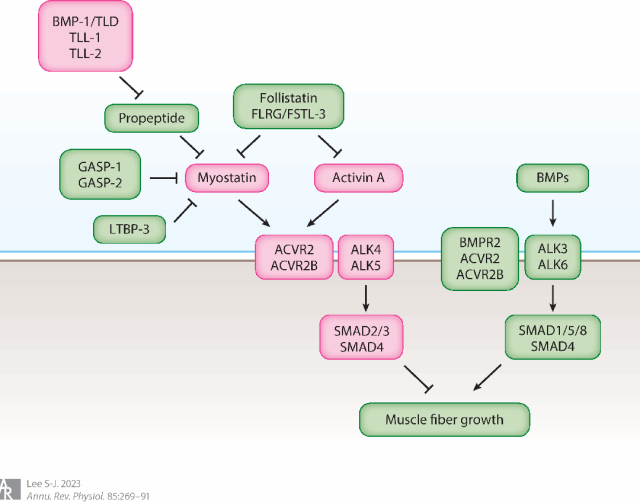
圖3 肌肉生長抑制素 (MSTN)、激活素 A 和骨形態發生蛋白 (BMP) 調節肌肉質量[4]。綠色中顯示的成分會誘導肌肉生長,粉色中顯示的成分會抑制肌肉生長。
03 治療潛力
GDF-8的一個主要生理功能就是調節肌肉與脂肪之間的整體代謝平衡[9],其信號通路是極具吸引力的藥物靶點。因其是進化遺留的“全局調控”系統,針對性干預被認為相對安全。目前,針對該通路的多種抑制劑(如抗體、受體誘餌劑等)已在眾多臨床適應癥中展開探索,旨在逆轉由肌營養不良、衰老、癌癥、肥胖等多種病因導致的肌肉萎縮,潛力巨大。
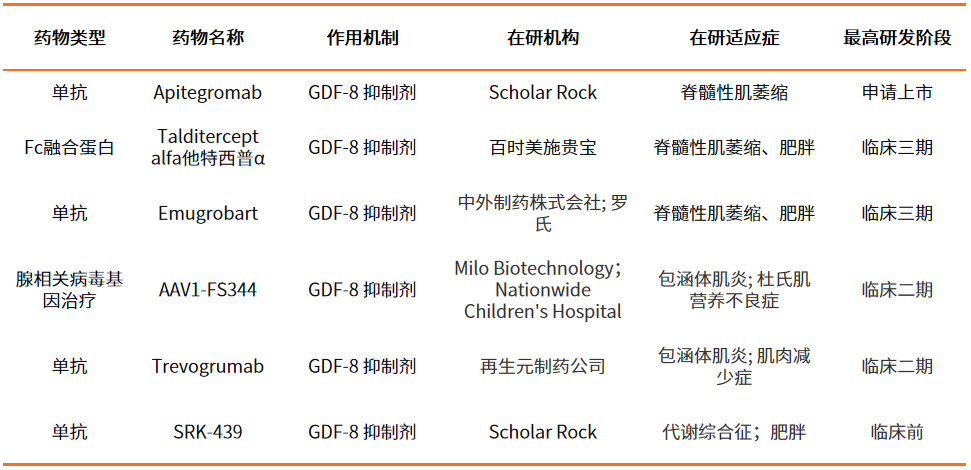
表1 靶向GDF-8藥物開發情況
近岸蛋白提供經過Cell/ELISA/BLI等多種方法驗證的GDF-8系列產品,活性好,純度高,批間穩定,現貨供應,全力助您加速GDF-8靶點的新藥研發進程!
GDF-8經過細胞和ELISA活性驗證
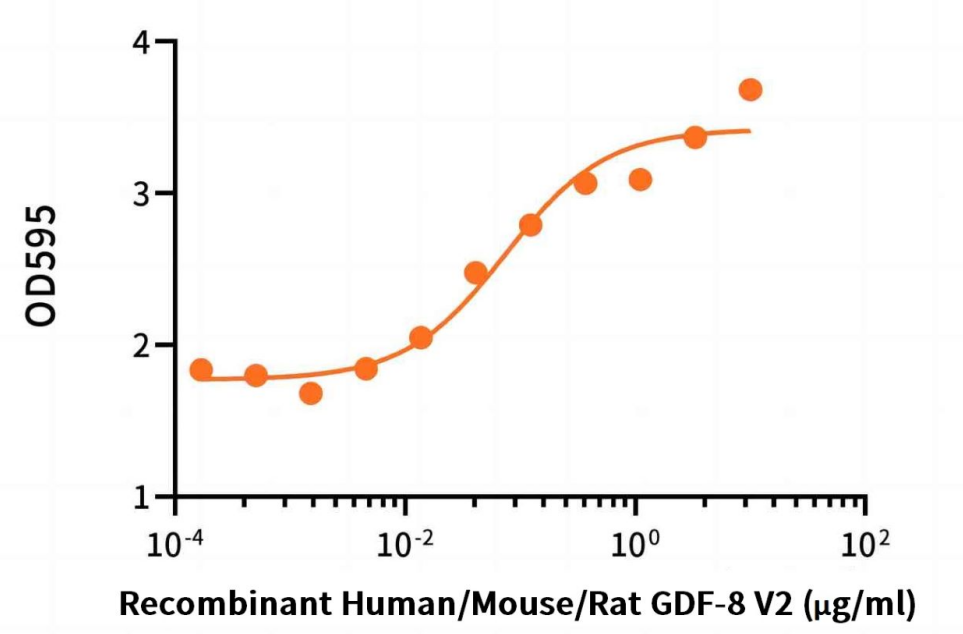
Measured by its ability to induce hemoglobin expression in K562 human chronic myelogenous leukemia cells. The ED50 for this effect is 74 ng/ml.
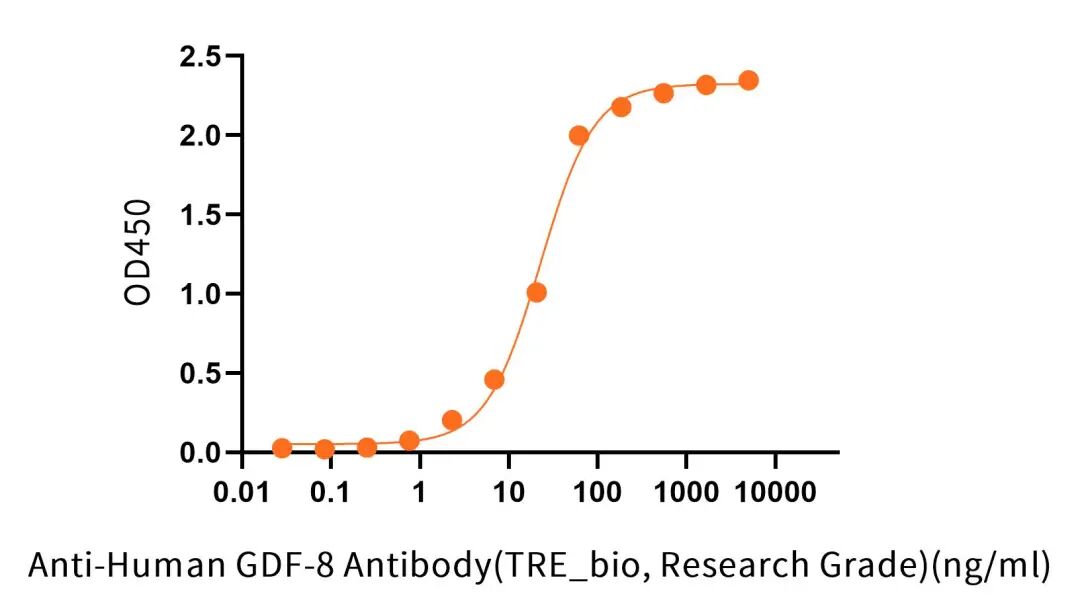
Immobilized Recombinant Human/Mouse/Rat GDF-8 V2(Cat.No.:C46R) at 2 μg/ml (100 μl/well) can bind Anti-Human GDF-8 Antibody (TRE_bio, Research Grade)(Cat.No.:NC366). The ED50 of Anti-Human GDF-8 Antibody (TRE_bio, Research Grade)(Cat.No.:NC366) is 22.66 ng/ml.
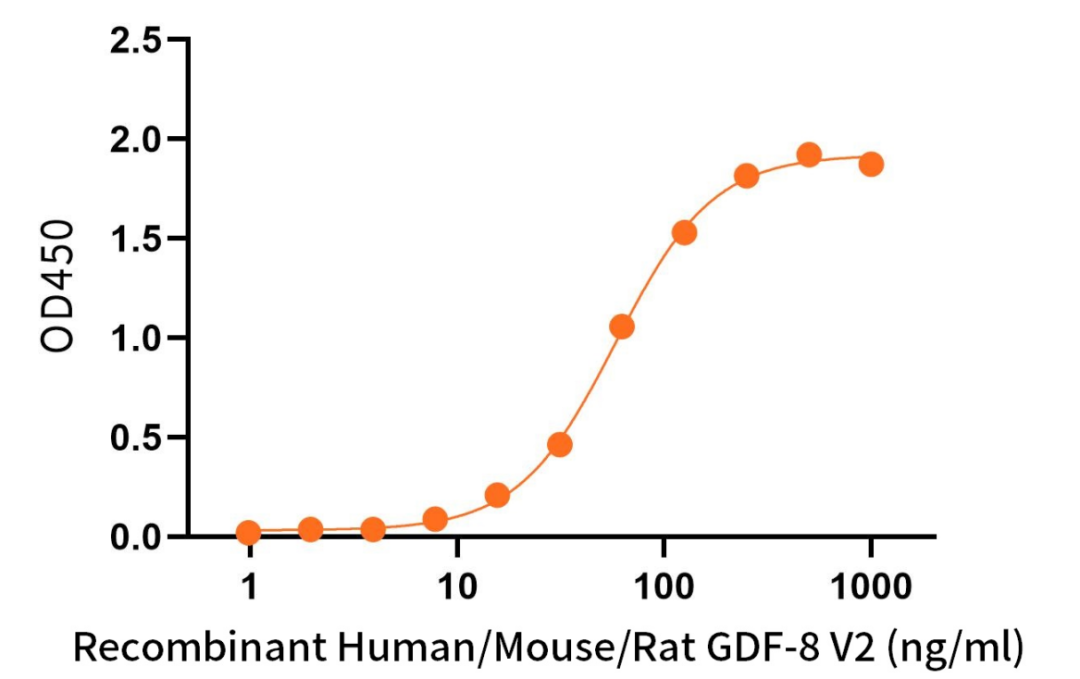
Immobilized Recombinant Human ACVR2B (C-6His)(Cat.No.:C302) at 2 μg/ml (100 μl/well) can bind Recombinant Human/Mouse/Rat GDF-8 V2(Cat.No.:C46R)*.
*: Biotinylated by NHS-biotin prior to testing. The ED50 of Recombinant Human/Mouse/Rat GDF-8 V2(Cat.No.:C46R) is 58.11 ng/ml.
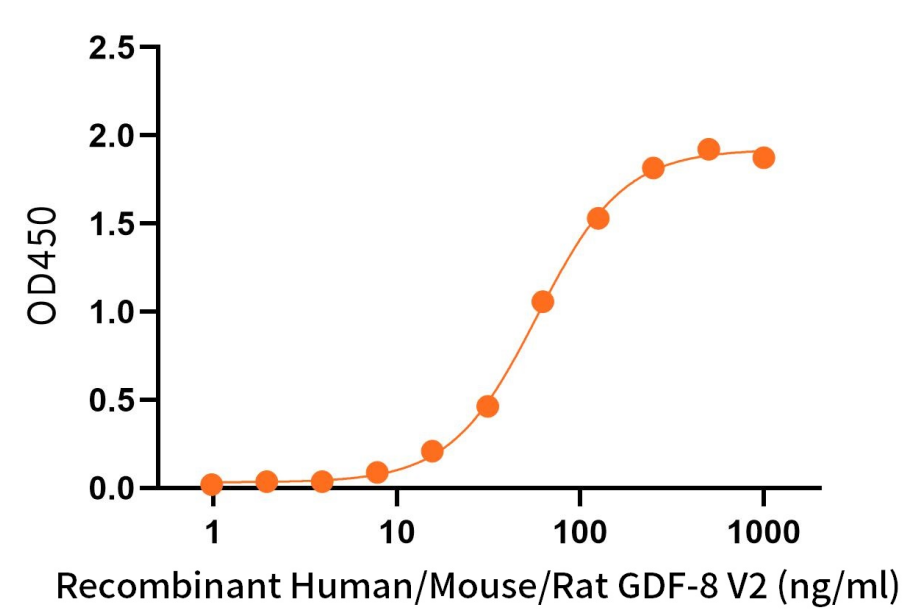
Immobilized Recombinant Human ACVR2B (C-Fc-6His)(Cat.No.:CX58) at 2 μg/ml (100 μl/well) can bind Recombinant Human/Mouse/Rat GDF-8 V2(Cat.No.:C46R)*.
*: Biotinylated by NHS-biotin prior to testing. The ED50 of Recombinant Human/Mouse/Rat GDF-8 V2(C46R) is 8.54 ng/ml.
Latent GDF-8經過BLI親和力驗證
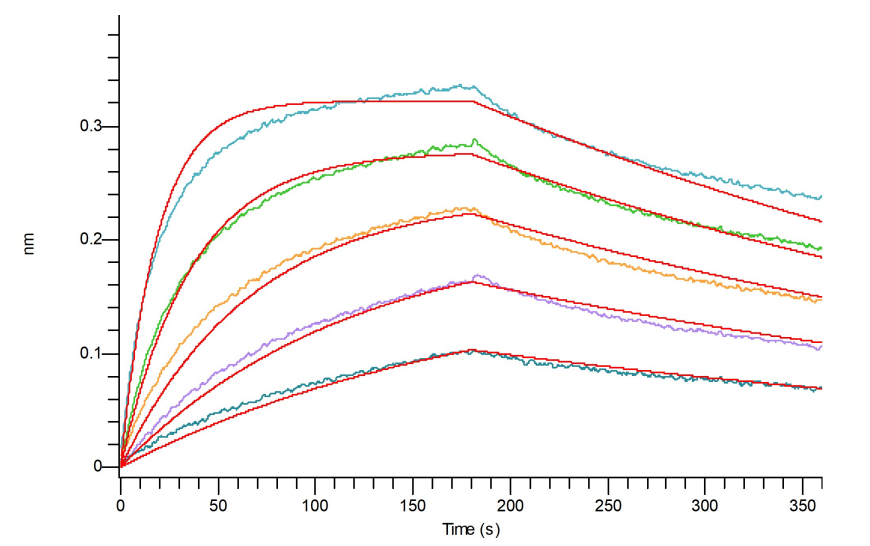
Loaded Anti-Human GDF-8 Antibody (API_bio, Research Grade) (Cat.No.:NC342) on Pro-A Biosensor, can bind Recombinant Human Latent GDF-8 (N-8His-Flag) (Cat.No.:C43S) with an affinity constant of 11 nM as determined in BLI assay.
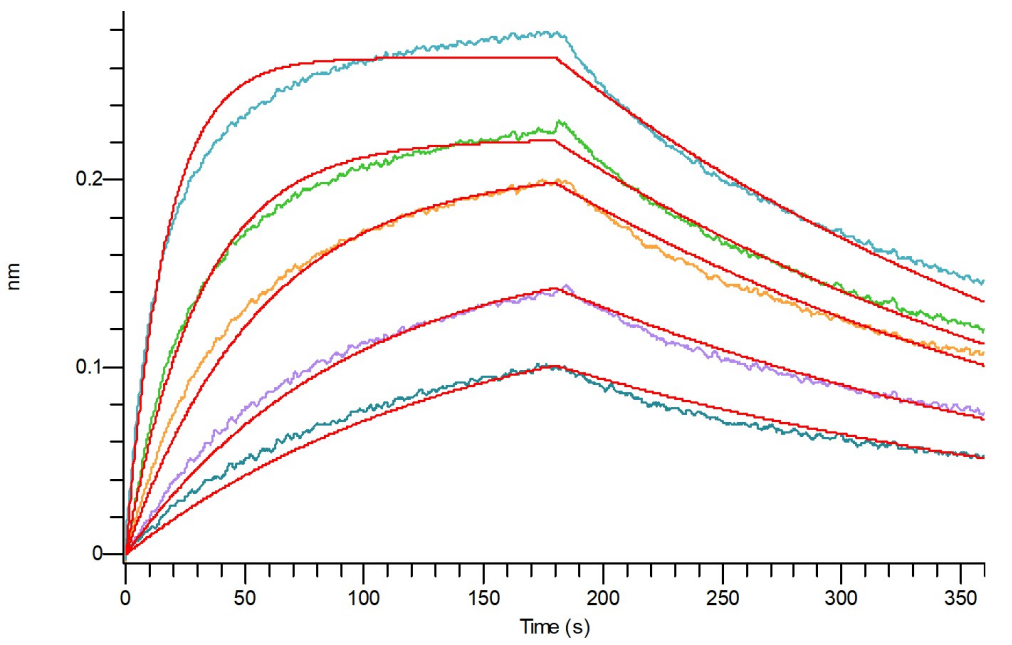
Loaded Anti-Human GDF-8 Antibody (API_bio, Research Grade) (Cat.No.:NC342) on Pro-A Biosensor, can bind Recombinant Mouse Latent GDF-8 (N-6His) (Cat.No.:C45S) with an affinity constant of 16.9 nM as determined in BLI assay.
GDF-8推薦產品
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
代謝相關產品
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
參考文獻
[1]SeJin L, Adam L, Yewei L, et al. Functional redundancy of type I and type II receptors in the regulation of skeletal muscle growth by myostatin and activin A. PNAS, 2020, 117 (49): 30907-30917.
[2]Jason W. M, Daniel G. G, José G. R, et al. GDF8 and activin A blockade protects against GLP-1–induced muscle loss while enhancing fat loss in obese male mice and non-human primates.Nature communication, 2025, 16, 4377.
[3]Alexandra C, McPherron, Ann M, Lawler et al. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member, Nature, 1997, 387, 83-90.
[4]SeJin L. Myostatin: A Skeletal Muscle Chalone. Annual review of physiology, 2023, 85, 269-291.
[5]McPherron A. C, Lee S. J. Double muscling in cattle due to mutations in the myostatin gene. PNAS, 1997, 94:2312457-61.
[6]Walker R. G, McCoy J. C, Magdalena C, et al. Molecular characterization of latent GDF8 reveals mechanisms of activation. PNAS, 2018, 115 (5): E866-E875.
[7]Rodgers B. D ,Ward C. W. Myostatin/Activin receptor ligands in muscle and the development status of attenuating drugs. Endocrine reviews, 2021, 43 (2): 329-365.
[8]Hoogaars W. M. H, Jaspers R. T. Past, Present, and Future Perspective of targeting myostatin and related signaling pathways to counteract muscle atrophy. Advances in experimental medicine and biology, 2018, 1088, 153-206.
[9]Lee S. Regulation of muscle mass by myostatin. Annual Review of Cell and Developmental Biology, 2004, 20 (1): 61-86.
